Neolamprologus pulcher and N. brichardi are two gregarious, altruistic, and social species that dominate parts of the rocky biotope in Lake Tanganyika. Their success is largely due to their strategy of cooperative breeding. Since the end of the 1990s, N. pulcher and N. brichardi have been increasingly regarded as synonymous; however, this is probably incorrect and appears to be based on inaccurate data and impulsive conclusions. This article details the argument to disapprove this synonymisation, including photographs of just over 100 geographical populations of N. pulcher and N. brichardi captured underwater along the Tanzanian shores of the Lake, usable as a variant identification guide.
Silaf Rocks
Derived from the Arabic word ‘zarafah’ (meaning giraffe in English), the name Silaf refers to the "towering" rocks that rise from the bottom of Lake Tanganyika at a depth of about 15 metres. Situated just outside of Mpimbwe Point of the larger Cape Mpimbwe, the underwater landscape of Silaf Rocks harbours a diverse array of cichlids, including an elegant variant of Neolamprologus pulcher.
 |
| Fig. 2. N. pulcher at Silaf Rocks, Cape Mpimbwe (map Fig. 52), depth 8 metres. |
Cooperative breeding
Neolamprologus pulcher is a cooperatively breeding cichlid that lives in permanent social groups, which usually comprise one breeding pair with up to 20 helpers of both sexes (Balshine et al., 2001), or several breeding individuals with even more helpers (Dierkes et al., 2008). In addition, observations indicate that also the helpers may take part in the spawning (Dierkes et al., 1999); however, this has been questioned by other researchers (Fitzpatrick et al., 2006). A large social group in the wild may comprise several neighbouring breeding groups, in total, constituting several hundred individuals. In the scientific literature, the cooperative and social behaviour in cichlids was first reported by Kalas in 1976, who studied the helping behaviour of N. brichardi (a species that is suggested to be synonymous with N. pulcher, but see below).
Instant recognition of family members
Members of species that display cooperative and social behaviours, such as N. pulcher, are known to be able to distinguish between familiar and unfamiliar fish, seemingly based on individual particular visual signals (Hert, 1985). Instead of body or fin related expressions, N. pulcher signals their identity primarily with facial colour patterns. Members of a group (a breeding or social group within a population) can perfectly and rapidly (faster that 0.5 sec) distinguish between familiar and unfamiliar individuals. The accuracy and speed by which this is conducted suggest a cognitive capacity for social recognition comparable to facial recognition by humans (Kohda et al., 2015).
 |
| Fig. 3. N. pulcher at Katondo (northern side), Cape Mpimbwe (map Fig. 52). |
 |
| Fig. 4. Plankton feeding N. pulcher at Kisala Bay (map Fig. 20), southern Tanzania. This species is not encountered in as large schools as is N. brichardi. See video of plankton feeding individuals here. |
Individual recognition: it is good to be different
When members of a group interact repeatedly, recognition of individuals is important to avoid expensive agonistic interactions and to maintain a stable social structure. Generally, individual recognition is more pronounced among highly social species, as in such groups, members need to be different (Tibbetts & Dale, 2007). An individually unique body pattern, even only minimal, may be a prerequisite for species that form large cooperating communities. In N. pulcher, the visual signals for individual recognition are restricted to the facial area around the eye or within the area from the snout to the gill cover.
Specific diversity of colour patterns
Two sympatric pomacentrid species (Pomacentrus amboinensis and P. moluccensis), with a very similar body appearance, including a solid yellow colouration, specifically and individually distinguish between each other by facial colour patterns (visible only in the ultraviolet spectrum), suggesting that facial colour pattern as an important character in species recognition (Kohda et al., 2015). This especially seems to apply to the Lake Tanganyika cichlids, which show a great diversity of colour patterns among populations and species, including the divergence in visual signals.
Isolation develops specificity
Speciation may occur in any geographical context, but is thought to be more common in allopatry, where mutation and random genetic drift might suffice. On the other hand, in sympatry, where selection is actively driven, the tempo of speciation is thought to be faster (Gante & Salzburger, 2012). In a DNA study of N. pulcher and N. brichardi, Duftner et al. (2007) found a complete lack of shared haplotypes (collections of gene variants that are usually inherited together) between populations of the same or different phenotypes (variants). They concluded that these findings indicate high genetic structures, reflecting a strong philopatric behaviour, meaning that members of the species will remain in close proximity to their birth place throughout their lives (Stiver et al., 2004). Consequently, it is likely that populations referred to as N. pulcher and N. brichardi may evolve into different variants and even distinct species, because speciation by means of geographic isolation is the most common mode of speciation. In addition, diversification of the colour pattern is often generated and maintained by natural or sexual selection (as opposed to random genetic drift).
 |
| Fig. 5. Kansombo (map Fig. 52 and here) has three smaller bays, named ‘The Large’, ‘The Medium’ and ‘The Small’. When we are referring to Kansombo, mostly this is the rocky stretch between the medium and small bay (Kansombo Kati Bay and Kansombo Ndogo Bay). This location is most beautiful and serene, and was the first proper dive site we visited back in 1989, after setting up our fish station at Katoba (adjacent to Udachi Village). Cape Mpimbwe is seen in the far distance, some 20 km south. |
 |
| Fig. 6. N. pulcher at Kansombo (map Fig. 52), at 20 metres depth, where the stone habitat transitions to sand. This variant is found throughout this coast north to Karema. |
 |
| Fig. 7. During the dry season in Tanzania, the leaves turn yellow and red before falling off, much like during autumn in Europe. The picture was captured in June 2007 and shows the woodland at Kansombo, 20 km north of Cape Mpimbwe (map Fig. 52 and here). |
Initial synonymisation
For many years, both N. pulcher and the similarly looking N. brichardi were widely accepted as two distinct species with validly published names. However, during the end of the 1990s some authors of scientific papers began to question this view and treated them as synonymous, with N. brichardi as a junior synonym of N. pulcher, for example, see Balshine-Earn et al. (1998) and Dierkes et al. (1999). Following the repeated synonymisation in scientific papers, Barlow (2000: 211) concluded that N. pulcher and N. brichardi pertain to one and the same species.
Behavioural similarity
In favour of the synonymisation is Dierkes et al. (1999), who questioned the view of treating them as two distinct species. They argued that the classification of them as two distinct species is invalid, primarily based on the authors’ field and laboratory observations on the behaviour and social system of the two species (N. brichardi from Burundi and N. pulcher from Zambia), of which are generally known to be identical, or nearly so. They included a systematic study in which 13 morphological characters were measured and statistically compared without finding strong support for the separation into two species. They also pointed out that Poll (1974) included only one specimen of N. pulcher in the comparison of morphometric characters. However, the original description of N. pulcher and N. brichardi as distinct taxa included 7 and 13 specimens respectively. Furthermore, as Poll (1974) declared, they do not differ much in meristic characters, and we may add, nor in measurements. However and more importantly, the original descriptions (Trewavas & Poll, 1952) also included details of the facial colour patterns, which are quite distinct and easily separable, so with a few exceptions, any population in the lake is easily classified as either N. pulcher or N. brichardi.
Invalid argument
Two similar species with different structures may be perfectly distinct species and classified as such despite exhibiting the same, or nearly the same behaviour in an identical, or almost identical ecological context. Hence, the claim by Dierkes et al. (1999) that the behaviour is an argument in favour of synonymisation seems to fall short.
 |
| Fig. 9 (left). N. pulcher at Katili Bay, southern side of Lwazi River (map Fig. 20). Fig. 10 (right). N. pulcher north of Kalepa (map Fig. 20). |
 |
| Fig. 11 (left). N. pulcher at Kalepa Village (map Fig. 20). Fig. 12 (right). N. pulcher at Kalepa Island (map Fig. 20). |
 |
| Fig. 13 (left). N. pulcher at Kazomba Point (map Fig. 20). Fig. 14 (right). N. pulcher at Molwe (map Fig. 20). |
 |
| Fig. 15 (left). N. pulcher at Polombwe Bay (map Fig. 20). Fig. 16 (right). N. pulcher at Muzi (map Fig. 20). |
 |
| Fig. 19. N. pulcher at Kapere, the last rocky habitat in southern Tanzania before Kalambo River, the border with Zambia (map Fig. 20). |
 |
| Fig. 20. Lake Tanganyika map, showing the localities mentioned in this article. Name references: Government of Tanzania, official maps and African Diving, lake survey 2007/2008. For a more detailed map over the southern Tanzanian shores of Lake Tanganyika, please see Tanganika Magazyn no. 17, page 22. |
 |
| Fig. 22. N. pulcher in Lamvya Bay, Samazi (Fig. 21 and map Fig. 20). This bay is protected from inclement weather by almost overlapping land strips (one being Cape Finga) blocking the entrance. |
 |
| Fig. 24. N. brichardi at Fulwe Rocks (map Fig. 20), an isolated habitat, located 2 km off the coast of Wampembe Village. |
 |
| Fig. 25. N. brichardi at Kungwe Point (map Fig. 104). |
Phylogeography
Furthermore, a phylogeographic study mapping the geographic distribution of mitochondrial genes was interpreted as to reflect taxonomic classification of N. pulcher and N. brichardi (Duftner et al., 2007). Similar phylogenetic studies have been made in the past, of which some have involved mitochondrial genes of the genus Tropheus (for example, see Sturmbauer and Meyer, 1992; Baric et al., 2003; Sturmbauer et al., 2005). These studies claimed to confer an improvement of the taxonomic classification: “The molecular phylogeny is in partial conflict with the present taxonomy. We suggest a revision of the taxonomy of Tropheus, which should include molecular data rather than rely solely on taxonomic characters like coloration or the number of spines in the anal fin that are currently used” (Sturmbauer & Meyer, 1992). Obviously, molecular data may aid in taxonomic classification; however, since phylogeography is a branch of genetics that concerns the relationship between gene genealogies and geography, in reality, it studies related genes rather than related organisms (Avise, 2000; Kullander, 2004). Using fast-evolving genes, particularly mitochondrial genes, phylogeography is not a genealogy of species. Occasional gene genealogies should perhaps ideally be regarded as complementary and subordinate to more traditional taxonomic characters, such as colouration, and morphology of finnage. Unless the study is mapping geographic distributions of genetic lineages, molecular data may be in excess. Since the informational content of a genome is enormous (Avise, 2004), a few genes, which may have been conveniently or randomly chosen, or the mapping of particular haplotypes, should not be interpreted as pertaining, especially not in a superior way, to taxonomic classification. Furthermore, it is a well-established fact that molecular phylogenetic trees (gene trees) may accord very poorly with species trees (Pamilo & Nei, 1988). As stated by Stiassny (1997: 521) when addressing the molecular phylogenetic analyses of the Lamprologini: “[I]t seems that the analysis of different genetic markers has resulted in markedly different schemes of intrarelationships for these fishes.” Furthermore, Streelman et al. (1998) commented on similar analyses: “Moreover, there is a growing appreciation that mitochondrial DNA represents only a single gene genealogy and that the results from this single locus may not be representative of organismal evolution.” Seemingly, the study of Duftner et al. (2007) could just as well be in favour of non-synonymisation of N. pulcher and N. brichardi.
Asymmetric hybridisation
Duftner et al. (2007) used DNA sequences of the mitochondrial control region and found that the relatedness of these sequences (haplotypes) disagreed with species assignment based on facial colour pattern. The authors inferred that this indicated repeated parallel evolution of particular facial colour patterns. Ultimately, they concluded that N. pulcher and N. brichardi are one species. Convergent and parallel evolution of facial colour pattern seems indeed likely, at least within each of the major facial colour patterns (N. pulcher and N. brichardi). However, they have largely omitted the opportunity to explain the results by means of hybridisation. Asymmetric hybridisation, where only one of the sexes of each species take part in the hybridisation, is thought to be common in such ecological context where similar, and possibly, closely related species co-exist and compete for territories, food and mates (Wirtz, 1999; Nevado et al., 2011). Such hybridisation may lead to the extinction of the inferior population (species) and a partial replacement of the mitochondrial genome of the extant population.
Hybrid origin
In the past, hybridisation was considered a rare occurrence, leading to nothing but an evolutionary dead end, but nowadays, it is increasingly accepted as an important evolutionary force (Arnold, 1992; Seehausen, 2006). As an example, N. marunguensis, a close relative of N. pulcher and N. brichardi, is said to be of hybrid origin, based upon the findings of two highly divergent mitochondrial lineages, and evidenced incongruity among mitochondrial and nuclear DNA sequences from samples of N. marunguensis (Salzburger et al., 2002). Moreover, the two species that appear to have been involved in the hybridisation are referred to as N. olivaceous-like and N. helianthus-like (Salzburger et al., 2002). Unravelling hybrid origin of species require particular procedures of investigation. Following such procedures, many more species will no doubt be unmasked.
Incomplete lineage sorting
Furthermore, rapid radiation and ‘incomplete lineage sorting’ (Doyle, 1992; Maddison, 1997), which is the uneven distribution of gene variants during segregation events of populations, are known to generate similar patterns of genetic variation as hybridisation and may also explain the occurrence of related gene variants in populations of N. pulcher and N. brichardi. Both were presented as explanations for similar results (conflicts between gene trees and species trees) from phylogeographic studies of the genus Tropheus and related genera (Koblmüller et al., 2010; Karlsson & Karlsson, 2015a).
Indication of hybridisation
Duftner et al. (2007) identified four major clades of mitochondrial DNA (mtDNA) sequences, one of which grouped N. pulcher from south of Lwazi River (southern Tanzania) and N. brichardi from north of Lwazi River. The authors suggested that the N. brichardi phenotype evolved from the N. pulcher phenotype, something which they believe has happened repeatedly and independently in the lake. However, what they probably do not know is that slightly farther north of Lwazi River there is an area with a few populations that comprise facial colour patterns which seem to be a mixture of both species. We believe that this facial colour pattern is not a transitional pattern, but rather, an indication of hybridisation. Probably, not just these populations are of hybrid origin, but also additional neighbouring populations, such as the ones found north and south of Lwazi River.
In addition, the Korongwe Bay area appears to be yet another locality of hybridisation. While a large northern part of Cape Mpimbwe, as well as the area farther north to Karema, harbours N. pulcher (Fig.3), and the area between Kampemba Point and Isaba Point harbours N. brichardi (Fig. 53), the localities in between, including Korongwe Bay and the southernmost part of Cape Mpimbwe, harbour slightly variable and mixed populations with characteristics of both species (Fig. 26), such as a shorter, or less distinct opercular stripe, of which upper part is yellow, reminiscent of N. brichardi, and a postorbital stripe curving downwards combined with a yellow eye ring, reminiscent of N. pulcher (see more below), indicating possible historical hybridisation between the two species. Furthermore, in Korongwe Bay we have found indications of hybridisation between additional species, such as between Tropheus sp. “Mpimbwe” and T. sp. “Kipili”, and between Eretmodus marksmithi and E. cyanostictus. Moreover, Korongwe Bay is not only a locality where species hybridise, or may have hybridised but also a geographical endpoint for the distribution of species, such as Petrochromis sp. “Ikola” and Neolamprologus furcifer (Udachi form, see Kullander et al., 2014a, for which Kampemba Point is the southernmost distribution), in addition to the four aforementioned species.
In addition, the Korongwe Bay area appears to be yet another locality of hybridisation. While a large northern part of Cape Mpimbwe, as well as the area farther north to Karema, harbours N. pulcher (Fig.3), and the area between Kampemba Point and Isaba Point harbours N. brichardi (Fig. 53), the localities in between, including Korongwe Bay and the southernmost part of Cape Mpimbwe, harbour slightly variable and mixed populations with characteristics of both species (Fig. 26), such as a shorter, or less distinct opercular stripe, of which upper part is yellow, reminiscent of N. brichardi, and a postorbital stripe curving downwards combined with a yellow eye ring, reminiscent of N. pulcher (see more below), indicating possible historical hybridisation between the two species. Furthermore, in Korongwe Bay we have found indications of hybridisation between additional species, such as between Tropheus sp. “Mpimbwe” and T. sp. “Kipili”, and between Eretmodus marksmithi and E. cyanostictus. Moreover, Korongwe Bay is not only a locality where species hybridise, or may have hybridised but also a geographical endpoint for the distribution of species, such as Petrochromis sp. “Ikola” and Neolamprologus furcifer (Udachi form, see Kullander et al., 2014a, for which Kampemba Point is the southernmost distribution), in addition to the four aforementioned species.
Replacement of mtDNA
In the evolutionary past, populations of migrating N. brichardi have likely dispersed into areas of N. pulcher. Following asymmetric hybridisation events with males of N. brichardi and females of N. pulcher, in combination with reproductive backcrossing of hybrids into the population of N. brichardi, the facial colour pattern has largely remained the same (a T-bar as in N. brichardi), but the mtDNA has been replaced by that of N. pulcher. Possibly, N. brichardi may initially have evolved as a northern specific representative of N. pulcher, or vice versa. Alternatively, their similarity is potentially by convergence. Nevertheless, the most recent common ancestor of all the princess-like species, such as N. pulcher, N. brichardi, N. splendens, etc., may be N. savoryi, since it appears as the least advanced morphologically, behaviourally and ecologically.
 |
| Fig. 28. N. brichardi at Kalyeza Bay (map Fig. 20), located on the northern side of the larger Kala Bay. |
 |
| Fig. 30. N. pulcher photographed along the steep rocky coastline between Katili and Kalepa villages (map Fig. 20). |
 |
| Fig. 31. N. pulcher at Kantalamba (map Fig. 20). |
 |
| Fig. 32. N. brichardi at Mvuna Island, Kipili (map Fig. 52). |
 |
| Fig. 35. N. brichardi at Nkondwe Island, Kipili (map Fig. 52). |
 |
| Fig. 36. N. brichardi at Kerenge Bay, Kerenge Island (map Fig. 52). |
 |
| Fig. 37. N. brichardi at Kerenge Bay, Kerenge Island (map Fig. 52). This species could be encountered in large schools, containing up to 1000 individuals. |
 |
| Fig. 38. Kerenge Village is located along the eastern side of Kerenge Island (map Fig. 52), the largest of eight islands in the Kipili archipelago, and the main fishing community. |
 |
| Fig. 39. N. brichardi at Kerenge Bay, Kerenge Island (map Fig. 52). It is not unlikely that the Kipili populations could be genetically diagnosable from N. brichardi. |
 |
| Fig. 41 (left). N. brichardi at Mwila Island, Kipili (map Fig. 52). Fig. 42 (right). N. brichardi at Mlowa Point, Kipili Mainland (map Fig. 52). |
 |
| Fig. 43 (left). N. brichardi at Kasisi Island, Kipili (map Fig. 52). Fig. 44 (right). N. brichardi at Lupita Island, Kipili (map Fig. 52). |
 |
| Fig. 45 (left). N. brichardi at Mbofula Points (map Fig. 52). Fig. 46 (right). N. brichardi at Twiu Point (map Fig. 52). |
 |
| Fig. 47 (right). N. brichardi at Mtosi Point (map Fig. 52). Fig. 48 (right). N. brichardi at Chamepa Point (map Fig. 52). |
 |
| Fig. 49 (left). N. brichardi at Pinga Point (map Fig. 52). Fig. 50 (left). N. brichardi at Ninde village (map Fig. 52). |
 |
| Fig. 51. N. brichardi at Kilingwe Point (map Fig. 52). |
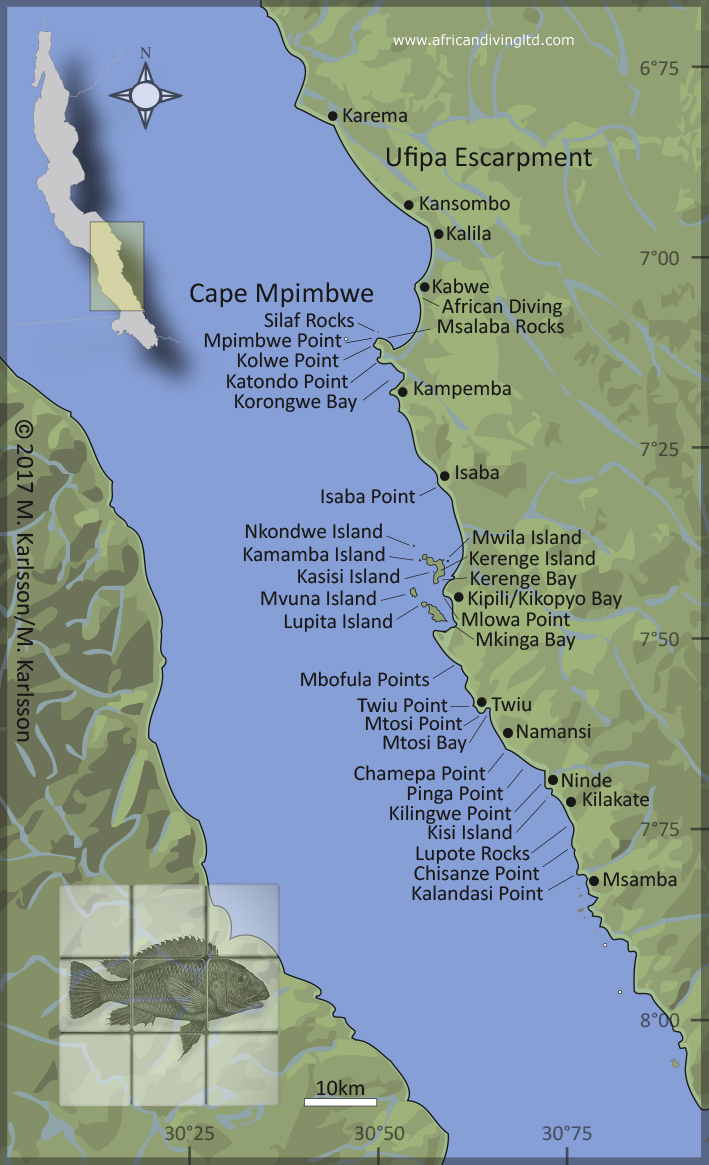 |
| Fig. 52. Lake Tanganyika map, showing the localities mentioned in this article. Name references: Government of Tanzania, official maps and African Diving, lake survey 2007/2008. For a more detailed map over the southern Tanzanian shores of Lake Tanganyika, please see Tanganika Magazyn no. 17, page 22. |
Remarkable taxonomic merger
It is rather remarkable to see that this phylogeographic study of Duftner et al. (2007) has resulted in a taxonomic merging of N. pulcher and N. brichardi. Few, if any, scientists and hobbyists would have accepted a merging of distinct Tropheus species that happen to have a few related genes. The study of Duftner et al. (2007) is a phylogenetic study in gene hierarchies and has little to do with taxonomic classification.
Diagnostic princess characters
Instead of merging N. pulcher and N. brichardi, a further split would seem more plausible. This would partly be based on colouration and morphology, such as the facial colour pattern, which is a widely accepted differentiating character among cichlid species such as N. pulcher and N. brichardi (Smith, 1995). Further useful characters are the number of gill rakers and number of scales in a lower transverse row, both which were used by Brichard (1989: 534–536) to diagnose several new princess-like species. Several more potentially differentiating characters are available, see below. In addition, the juvenile body colour pattern may also suffice as a diagnostic character. For a summary of the standardised characters used in the diagnosing of the princess-like species, see Verburg and Bills (2007), and Karlsson and Karlsson (2017b).
The facial colour pattern of N. pulcher
The facial colour pattern of N. pulcher was described as follows:
“A dark streak behind eye, curving downwards along posterior edge of preoperculum; a parallel streak on operculum” (Trewavas & Poll, 1952: 7). This pattern may be referred to as the ‘parallel bars’. The type specimens were collected by Cuthbert Christy in 1926–1927. According to Trewavas and Poll, they are from an unknown locality in Lake Tanganyika, which, incidentally, largely all fishes collected by Cuthbert Christy seem to be. Yet, the species is identifiable due to the diagnostic information specified in the original description. In a subsequent publication, however, the type locality is indicated as Kasanga (Maréchal & Poll, 1991: 289), a statement in which Konings (2015: 118) and Eschmeyer et al. (2017) concur.
The facial colour pattern of N. brichardi
The facial colour pattern of N. brichardi was described as follows:
“An oblique bar extending backwards and downwards from eye on to operculum, absent in some specimens; a dark vertical bar on posterior part of operculum” (Trewavas & Poll, 1952: 6). This pattern may be referred to as the ‘T-bar’ (cf. Konings, 1998: 75, 2015: 118; Duftner et al., 2007: 707). The type specimens were collected by Max Poll and the Belgian hydrobiological mission in 1946–1947. Most of the specimens are from just north of Lubulungu River, at about the centre of Mahale Mountains.
A yellow eye ring and a naked cheek
There are two additional and potentially differentiating characters that appear to have been omitted by researchers, such as Dierkes et al. (1999) and Duftner et al. (2007). The first is the colouration around the eye, which seems to be linked with the facial colour pattern. In N. pulcher, with the ‘parallel bars’, there is always a yellow ring around the eye (sometimes only slightly visible). Normally, the yellow colour is more evident in the upper part of the eye and even extends into the iris. Conversely, in N. brichardi, with the ‘T-bar’, there is never a yellow ring; the colouration is the same as the body. The second character is the cheek, i.e., the facial area immediately below the eye. Besides a suborbital blue stripe, N. pulcher displays a naked cheek with the complete absence of supplementary markings of any colour. In contrast, N. brichardi displays, in addition to the suborbital blue stripe, an intriguing and irregular, frequently strongly colour-contrasting expression of usually blue and/or yellow straight and curved lines with occasional dots. These two characters (the potential colouration around and below the eye) are correlated with the facial colour pattern (the opercular colour pattern of either the ‘double bars’ or the ‘T-bar’) and seem to be additional arguments in favour of accepting N. brichardi as a "valid" (distinct) species (the initial argument being a distinct and easily separable facial (opercular) colour pattern).
 |
| Fig. 54. N. brichardi at Bulu Point (map Fig 104). |
 |
| Fig. 55. N. brichardi at Chamepa Point (map Fig 52). |
 |
| Fig. 56. N. brichardi at Tundu, Kala Bay (map Fig. 20). |
 |
| Fig. 58. N. brichardi at Lwasase Bay (map Fig. 20). |
 |
| Fig. 59. N. brichardi at Kisi Island (map Fig. 52). |
 |
| Fig. 60 (left). N. brichardi at Lupote Rocks (map Fig. 52). Fig. 61 (right). N. brichardi at Yamsamba Island (map Fig. 20). |
 |
| Fig. 62 (left). N. brichardi at Lwilwi Island (map Fig. 20). Fig. 63 (right). N. brichardi at Popo Point (map Fig. 20). |
 |
| Fig. 64. N. brichardi at Kashia Island (map Fig. 20). |
The largest communities and the highest feeding rate
Furthermore, it is occasionally thought that N. brichardi forms larger communities than N. pulcher, which, if confirmed, would suggest that N. brichardi has a higher social and communicative ability than N. pulcher, which in turn would be an additional separating character. Brichard (1989, cited in Verburg and Bills, 2007: 33), reported that N. brichardi gathers in large feeding communities that can consist of 100,000’s of individuals. Over the years, we have found several localities (such as Maleza Island, Kerenge Island, Katumba Point, Halembe, Cape Kabogo, Kigoma, etc.) with very large communities of N. brichardi, but never any localities with such large communities of N. pulcher. In addition, N. brichardi may have a higher feeding rate than that of N. pulcher. In the Kigoma area, N. brichardi is observed to have the highest feeding rate among the princess-like species (N. brichardi, N. walteri, N. chitamwebwai, and N. savoryi) (Verburg & Bills, 2007).
A deeper body and conspicuous dots on the scales
Moreover, two more characters may be mentioned which may further differentiate these two nominal species. Firstly, N. pulcher appears to have a deeper body than that of N. brichardi. This characteristic was already mentioned by Trewavas and Poll (1952: 2). It may be observed in an underwater video featuring the population of N. pulcher at Kitawe (between Samazi and Molwe in southern Tanzania) (Karlsson & Karlsson, 2017a: 17sec). Secondly, N. pulcher always, or very commonly, displays conspicuous orange, or red, tiny dots on the scales of the body (compare Trewavas and Poll, 1952: 7), which frequently form multiple (6–7) longitudinal series, so-called ‘dotted stripes’. This feature used to be regarded as typical of N. pulcher (Herrmann, 1985: 152). Furthermore, these dots may occasionally, at the populational level, be unusually oblong, which results in the series of dots apparently becoming coherent stripes and homogeneous lines (see, for example, fig. 29). In contrast, N. brichardi never displays red or orange dots, but, and on rare occasions only, dots that are yellow. When it does, these yellow dots form a subdued and less vivid network of both longitudinal and latitudinal series (fig. 32). However, these additional separating characters are temporal, pending a taxonomic study.
Characteristics of Neolamprologus olivaceous
Relating to the character of lateral dots or, more precisely, dots on lateral scales, N. olivaceous is commonly regarded as being characterised by such a feature, i.e. possessing about half a dozen longitudinal and lateral conspicuous dots that form coherent stripes; for example, see Verburg and Bills (2007: 28 and 36). However, Brichard (1989: 535) did not explicitly mention this characteristic in his original diagnosis of N. olivaceous, nor, as we interpret it, did he implicitly mention this characteristic. Initially, he mentioned that it “is similar to” N. pulcher. This is implicitly most likely with regard to the facial colour pattern, because further in the diagnosis he mentioned that “the postorbital brown stripe is faint”. He never mentioned anything about any dots on the lateral scales, neither in his diagnosis of N. olivaceous nor in his rediagnosis of N. pulcher (Brichard, 1989: 534–535). He did, however, mention in his rediagnosis of N. pulcher that this species has orange scale edgings of the sides, but only in relation to N. brichardi, of which scale edgings are stated to be less vivid (Brichard, 1989: 534). The negligence of the ‘multiple conspicuous horizontal stripes’ is remarkable, as such a characteristic, with which N. olivaceous has come to be associated, is quite conspicuous in itself. For a visualisation of the characteristic, see a picture (figs. 22, 29) and video sequence (Karlsson and Karlsson 2017: 27sec). Had N. olivaceous possessed it, Brichard would have mentioned it. Brichard (1989: 535) described N. olivaceous as “pale greenish beige” (hence its name), which is quite different from ‘multiple conspicuous horizontal stripes’. Furthermore, Brichard (1989) stated the scales of N. olivaceous to be delineated by faint brown margins. This latter descriptive statement is very reminiscent of the colour description of N. walteri: “Caudal margins of scales brown, produces a checkered pattern on the body” (Verburg & Bills, 2007: 32).
Type locality of N. olivaceous
Not only is the colouration of N. olivaceous of uncertain parameter, but the type locality is, too. Brichard (1989: 535) described it to be “in and around the Bay of Luhanga”. Luhanga Bay, which is in the very northwest of the lake, is possibly a misspelling of Lunangwa Bay. The type locality written on the holotype label of N. olivaceous is that of Lunangwa Bay, in the southwest of the lake (Verburg & Bills, 2007: 27). Besides Brichard (1989), a princess-like species conforming to N. olivaceous appears never to have been reported from Lunangwa Bay or from Luhanga Bay. However, except for the Tanzanian coastline, which has been investigated thoroughly during recent expeditions, such as our own in 2007–2008, much “of the coast line has not been extensively investigated, and there may be more undiscovered species within the complex” (Verburg & Bills, 2007: 27). This also includes the potential rediscovery of N. olivaceous and its possibly alternative type locality.
This issue with N. olivaceous is mainly a result of the information given in the literature, including Brichard (1989) and Verburg and Bills (2007). The actual type series of N. olivaceous has not been investigated.
 |
| Fig. 68 (left). N. brichardi at Kasola Mainland (map Fig. 20). Fig. 69 (right). N. brichardi at Kapumpuli Village (map Fig. 20). |
 |
| Fig. 70. Kasola Island protects the adjacent bay from stormy weather. Picture captured from within the bay. |
 |
| Fig. 71. N. pulcher at Mpando Point just south of Lwazi River (map Fig. 20). |
 |
| Fig. 72. N. brichardi at Fulwe Rocks (map Fig. 20). |
 |
| Fig. 73 (left). N. brichardi at Popo Rocks (map Fig. 20). Fig. 74 (right). N. brichardi at Kanena Point, Wampembe (map Fig. 20). |
 |
| Fig. 75 (left). N. brichardi at Namlimba Point, Wampembe (map Fig. 20). Fig. 76 (right). N. brichardi at Fulwe Rocks, Wampembe (map Fig. 20). |
 |
| Fig. 77 (left). N. brichardi at Sangalawe Point (map Fig. 20). Fig. 78 (right). N. brichardi at Natimba Point (map Fig. 20). |
 |
| Fig. 79 (left). N. brichardi at Izinga Island (map Fig. 20). Fig. 80 (right). N. brichardi at Lyela (map Fig. 20). |
 |
| Fig. 81. N. brichardi at Izinga Island (map Fig. 20). |
 |
| Fig. 82. Neolamprologus savoryi at Kambwimba (map Fig. 20). |
Hybridisation renders a non-cladistic phylogeny
There are a few populations of N. pulcher and N. brichardi in southern Tanzania that seem to display a mixture of these two nominal species (the facial colour pattern being a combination of both the ‘double bars’ and the ‘T-bar’), possibly indicating a natural transition from one variant to another, or, and more likely, natural hybridisation between two distinct forms (species). Furthermore, any of these populations may involve secretive and ancient hybridisation, i.e., hybridisation that does not easily show and involves reproductive backcrossing of hybrids into one or both of the parent species. This in turn should have resulted in introgression of the genome, and their evolutionary history becoming a network (reticulated phylogeny).
Translocation following water level fluctuation
Originally, N. brichardi was very likely restricted to the northern half of the lake, while N. pulcher was constrained, and still is, to the southern half. Following the dispersal events caused by a historically fluctuating lake level, fish populations were naturally translocated. Judging from the present biogeographic map of these species, it seems that populations of N. brichardi have eventually migrated south, into the southern half of the lake, creating an alternating occurrence of N. pulcher and N. brichardi with occasional and discernible hybrid populations in between. These hybrid populations, which may be referred to as populations of hybrid origin, seem to be well established, both structurally and ecologically. They have a function within their environment. Being distinct and separable, they may perhaps better be referred to as established species, such as the populations at Nkonkonti Point and the nearby Nakiwumbu Rocks, both localities north of Lwazi River (Karlsson & Karlsson, 2014).
Morphological clines between Neolamprologus pulcher and N. brichardi do not exist
While some areas harbour hybrid populations, others display sharp transitions between the two forms (N. pulcher and N. brichardi). Dierkes et al. (2005) argue that the lake-wide distribution of these two nominal species is interconnected by populations showing clinal morphological variation, which in turn is interpreted as to suggest that they are the same species. However, smooth, or clinal, transition from one form (species) to the other occurs only very rarely (in few geographical areas), and when it does, the facial colour pattern is not a pattern in transition from one to the other, but a rather irregular combination of both. Such a pattern is more indicative of a hybridisation event than a cline.
Allopatry is incorrectly interpreted as conferring synonymy and conspecificity
The assumption that N. pulcher and N. brichardi are the same seems to be driven by the fact that these two nominal species have never been found together (Dierkes et al., 2005; Duftner et al., 2007). It appears that they are strictly allopatrically distributed; hence, they never co-exist. As a result, they might be interpreted as geographical variants of the same species. However, the geographical aspect should be left out when "validating" species, i.e. examining wheter they are distinct or not. A unique set of intrinsic characteristics is what matters, such as a distinct colour pattern, which, incidentally, is the most common character used in diagnosing species. The hybrid populations excluded, any population in the lake is quite easily classified, based on the facial colour pattern and the eye ring, as either N. pulcher or N. brichardi.
 |
| Fig. 86. Nakiwumbu Rocks are located on the eastern side of Lwasase Bay (map Fig. 20). |
 |
| Fig. 87 (left). N. brichardi at north of Lwasase Point (map Fig. 20). Fig. 88 (right). N. brichardi at Nakiwumbu Rocks (Fig. 86, map Fig. 20). |
 |
| Fig. 89 (left). N. brichardi at Lwasase Bay, western side (map Fig. 20). Fig. 90 (right). N. brichardi at nearby Nkonkonti Point (map Fig 20). |
 |
| Fig. 91. N. brichardi at Lwasase Bay, eastern side (map Fig. 20). |
 |
| Fig. 92. N. brichardi at Lusekese Bay, a small bay at the northern side of the larger Kala Bay (map Fig. 20). |
 |
| Fig. 93. N. brichardi at Tundu, Kala Bay (map Fig. 20). |
 |
| Fig. 94 (left). N. brichardi at Kalyeza Point (map Fig. 20). Fig. 95 (right). N. brichardi at Kitango Rocks, Kala Bay (map Fig. 20). |
 |
| Fig. 96 (left). N. brichardi at Mikongolo Island (map Fig 20). Fig. 97 (right). N. brichardi at Kalala Island, the northernmost and second smallest islands in the Kala archipelago (map Fig. 20). |
 |
| Fig. 98. N. brichardi at Maleza Island (map Fig. 20). This was one of the first variants we collected back in 1990, due to its great abundance at the island. |
 |
| Fig. 101 (left). N. brichardi at Singa Island (map Fig. 20). Fig. 102 (right). N. brichardi at Kala Island (map Fig. 20). |
 |
| Fig. 103. Singa Island, northern side. From here, an underwater ridge runs 500 meter north to Kala Island, connecting the underwater habitats of the islands (map Fig. 20). |
 |
| Fig. 104. Lake Tanganyika map, showing the localities mentioned in this article. Name references: Government of Tanzania, official maps and African Diving, lake survey 2007/2008. For a more detailed map over the southern Tanzanian shores of Lake Tanganyika, please see Tanganika Magazyn no. 17, page 22. |
 |
| Fig. 105. N. brichardi at Lusungura Point (map Fig. 104). |
 |
| Fig. 106. N. brichardi north of Segunga Bay (map Fig. 104). |
 |
| Fig. 107. N. brichardi at Katumba Point, depth 10 metres (map Fig. 104). |
 |
| Fig. 108. N. brichardi at Ifala Village, located just south of Mkuyu Village (map Fig. 104). |
 |
| Fig. 110 (left). N. brichardi at Cape Kabogo (map Fig. 104). Fig. 111 (right). N. brichardi at a rocky outcrop, 4 km north of Segunga Bay (map Fig. 104). |
 |
| Fig. 112 (left). N. brichardi at Halembe (map Fig. 104). Fig. 113 (right). N. brichardi at Bulu Point (map Fig. 104). |
 |
| Fig. 114 (left). N. brichardi at Karilani Island (map Fig. 104). Fig. 115 (right) N. brichardi at Miyako Point (map Fig. 104), probably the true type locality of N. brichardi. |
Neolamprologus brichardi in the Kipili area
The Kipili area harbours a set of populations that we classify as N. brichardi. However, these populations lack a particular feature apparent in all other populations of N. brichardi (and absent in all populations of N. pulcher), that is the rather typical colour pattern on the cheek (see above for a description). The aforementioned N. brichardi populations display either a naked cheek, or a cheek with a strongly reduced and faint colour pattern. Particularly, those populations with a naked cheek are found in the northern part of the Kipili area, and those with a reduced pattern in the southern part, the latter which extends, incidentally, south to Mtosi Bay. Furthermore, all populations, including both the northern and southern populations, appear to have a shorter body and not as elongated caudal, anal, and dorsal-fin lobes and filaments, compared to more regular N. brichardi found elsewhere. Despite these slightly morphological and colourational deviations, we continue to refer to the Kipili populations as N. brichardi. Alternatively, they could be referred to as N. cf. brichardi.
The peculiar distribution occurrences of species and variants in the Kipili area
The Kipili area also harbours two distinct sets of populations of certain species. This phenomenon has previously been documented, for example, see Karlsson and Karlsson (2013, 2015b). It has been observed that recognisable populations are found in the five northernmost islands (‘the northern group’, i.e. Kamamba, Kasisi, Kerenge, Mwila, and Nkondwe Islands), different from those found in the three southernmost islands (‘the southern group’, i.e. Lupita, Mvuna, and Ulwile Islands). Within each group of islands, the populations of each species look similar but there may be striking differences in colouration, or sometimes even in the distribution of certain species between groups. For example, Ophthalmotilapia boops of the northern group of islands have a thick neon blue horizontal stripe on the posterior part of the flank, including parts of the caudal fin (the so-called ‘Neon Stripe’), while same species is uniformly black or dark brown at the southern group of islands. Regarding the congeneric O. sp. “Whitecap”, the northern variants exhibit an all black or dark brown colouration, and the southern variants a dark-blue orange colouration with a reddish or orange finnage (the so-called ‘Firefin’). Furthermore, at the northern islands Tropheus sp. “Kipili” displays yellow pectoral fins, but greyish hyaline at the southern islands. Moreover, this species has an overall dark brown adult colouration in the north, but dark brown with mostly bright yellow vertical stripes in the south. Petrochromis famula differs with regards to the dorsal, anal, caudal, and pelvic fins. At the northern islands (as well as farther north, probably to Isonga), these fins are either dark brown (the caudal fin), or light reddish beige. In addition, the dorsal fin has a dark brown margin. At the southern islands (as well as farther south, at least to Mtosi Bay), the fins are bright blue (the dorsal, anal, and pelvic fins), or dark brown blue (the caudal fin). In addition, there is a bright yellow margin in the dorsal and anal fin, and partial in the caudal fin. Neolamprologus cylindricus differs slightly, with thinner vertical light beige bars across the flank of the body at the northern islands than at the southern islands. Moreover, a few species exist only at either one of the island groups, being absent from the other. For example, within the Kipili area, Chalinochromis cyanophleps (Kullander et al., 2014b) {PDF}, N. furcifer and N. christyi are found only at the southern islands, while N. sp. “Eseki” and Lepidiolamprologus kamambae are encountered only at the northern islands. However, the latter species has been up to now only found at two of the northern islands (Kamamba Island and Kerenge Island), but could also potentially be located elsewhere on the northern islands and possibly farther north along the mainland, pending further investigations. These peculiar findings of species and variant distribution also relate to the mainland, where a northern-southern barrier appears to be somewhere south of the Kipili village (between Kikopyo Bay and Mkinga Bay); north of this barrier, the cichlid populations are similar to those at the northern islands, and vice versa.
Similar yet different
Despite the call for leaving the geographic aspect aside, one example with a geographical context is worthy of mention. Between Kapampa and Kiku in the DR Congo, there are populations that are usually referred to as N. brichardi. As it seems, they have never been explicitly reported to exist sympatrically with N. splendens. The latter is another cooperative breeding cichlid species with both species-specific and individual-specific facial colouration (Kohda et al., 2015). N. splendens have a facial colour pattern that looks quite similar to, yet distinct from, a few other species, among them the N. brichardi variant from this area, but not, however, N. pulcher (from any area). Nonetheless, irrespective of a non-sympatric existence, and all due to the diagnosable intrinsic characters, there is no doubt that N. brichardi and N. splendens are both distinct species. Furthermore, N. splendens may not be a typical species forming large communities, but neither is N. brichardi in this area. Moreover, the two species may indeed look similar. It seems as if Trewavas and Poll (1952: 6) may have misidentified specimens of N. splendens from Mwerazi as N. brichardi, as only N. splendens is known to occur at Mwerazi. In addition, Brichard’s (1989: 534) claim that N. brichardi and N. splendens are sympatric at Zongwe (near Mwerazi) seems to be incorrect.
New endeavour
Many interesting cichlids found along the coast of southern Tanzania were first located by our team in the early 1990s, following the establishment of our fish company in 1987, and the subsequent fish station near Kabwe Village (north of Cape Mpimbwe) two years later. Over the years, we have studied and become familiar with more than 200 different populations of N. pulcher and N. brichardi in the lake, of which, more than 100 were photographed during our expeditions in 2007–2008. Our first experiences with these kinds of fish were with the variants of N. brichardi from Kigoma, Maleza Island and Kerenge Island. The latter two were included in the consignment of cichlids to Åleds Akvarium in Sweden on 11 July 1991. A year later, we came across the beautiful variant of N. pulcher found at locations around Cape Mpimbwe (such as Silaf Rocks, Kolwe Point, Katondo Point, etc., and initially exported to Africa Cichlid Company in Marseilles, France, in December 1992). This variant displays conspicuous orange dots on the scales of the body and the facial colour pattern includes a speckled cyan bar between the regular parallel bars. Furthermore, there is a cyan horizontal streak below the eye, which extends ventrally. In our final stocklist from 24 August 2003, issued four months before we decided to discontinue collecting live fish, they were labelled Neolamprologus pulcher “Orange dots – Cape Mpimbwe”. Moreover, the variant was featured in a diving log and inventory guide to the cichlids of Cape Mpimbwe (Karlsson, 1998). Following our decision to discontinue with the collecting of live fish, and as a reference to the reason for it, we updated our website in 2004 with the following words: “New endeavour is the concern of Lake Tanganyika and its environment.”
 |
| Fig. 116. Mutant coloured individual, a so-called Kushangaza, of N. brichardi, photographed in southern Tanzania. However very rare, Kushangaza individuals are occasionally seen in N. brichardi, as well as in other Lamprologini. |
 |
| Fig. 117. A Kushangaza individual of N. brichardi, photographed in southern Tanzania. |
 |
| Fig. 118. An odd coloured individual of N. pulcher, photographed at Kitawe (map Fig. 20). The absence of a colour pattern on the cheek is one of the characteristic features of this species. |
 |
| Fig. 119 (left). N. pulcher at Molwe (map Fig. 20). Fig. 120 (right). N. brichardi at Namansi (map Fig. 52). |
 |
| Fig. 121 (left). N. pulcher at Kalubale, southern Tanzania (map Fig. 20). Fig. 122 (right). N. pulcher at Cape Finga, Samazi (map Fig. 20). |
 |
| Fig. 125. N. pulcher at the southern side of Kolwe Point, Cape Mpimbwe (map Fig. 52). |
Intriguing biogeographic pattern
The lamprologin species are estimated to be more than 5 million years old (Sturmbauer et al., 2010). Many geological and evolutionary events have happened over the years, causing species to form and spread, shrink and collapse, as well as hybridise. Concerning the historical procedure of these events, one can only speculate. Nevertheless, the current situation is an intriguing pattern of biotic distribution. How the present biogeographic pattern of the many populations of N. pulcher and N. brichardi has evolved is a mystery. However, this does not preclude the populations from being sortable and classified. We only need to realise that the pattern exist, not how it came to be.
Neolamprologus pulcher and N. brichardi are distinct species
In conclusion, based on the arguments and illustrations of more than 100 different populations presented in this article, N. pulcher and N. brichardi are undoubtedly two distinct species. Nevertheless, when taxonomically defining their inclusiveness, by clear diagnoses with differentiating characteristics, additional species may arise.
A species becoming a species group
Returning to the giraffe, which was mentioned in the beginning of this article, it was previously thought to be one widely distributed species with several subspecies. However, recent studies have demonstrated that giraffes may actually comprise at least six, and possibly as many as eleven separate and cryptic species (Brown et al., 2007; Beheregaray & Caccone, 2007), or, alternatively, four distinct species (Fennessy et al., 2016). The species show distinct and easily visible differences in characters such as body colour patterns and ossicones (horns). In addition, mtDNA and nuclear microsatellite DNA analyses of six species revealed that they are more genetically distinct than previously thought, and that some were separated from each other about 1.5 million years ago (Brown et al., 2007). Initially being a species, the taxon (Giraffa camelopardalis) is now a species group, comprising at least four species, including the additional three: G. reticulata, G. tippelskirchi, and G. giraffa (Fennessy et al., 2016).
Extinction in progress
In addition to the taxonomic work, conservational work is also urgently needed (IUCN, 2016; GCF, 2016a,b). Following a dramatic 36–40% decline from approximately 151,702–163,452 individuals in 1985 to 97,562 in 2015, the giraffe is now threatened with extinction (IUCN, 2016; Muller et al., 2016). Some of the suggested new species are down to a few thousand individuals (Muller et al., 2016). The unsustainably growing human population is having a very negative impact on most wildlife around the world. Illegal hunting, habitat loss and changes through expanding agriculture and mining, increasing human-wildlife conflict, and civil unrest are all pushing the giraffe towards extinction (IUCN, 2016). The giraffe species group is now listed as vulnerable and some of the species as endangered in the IUCN Red List (Muller et al., 2016). If nothing radical is done to protect the world’s wildlife, including the giraffe, it may very likely be irreversibly gone within a few years.
The End
Given the gloomy development of the natural world as we see today, the wildlife of Lake Tanganyika, including its cichlids, will also likely be extinct or critically endangered in the not too distant future.
A slightly shorter version of this article was published on African Diving Facebook on 11 March 2017.
Suggestion on how to cite this blog article:
Karlsson, M. and Karlsson, M. (2017) Neolamprologus pulcher and the analogy of N. brichardi. African Diving Blog, 18 June 2017. Available from: http://blog.africandivingltd.com/2017/06/neolamprologus-pulcher-and-analogy-of-n.html (accessed [day month year])
References:
Arnold, M. L. (1992) Natural hybridization as an evolutionary process. Annual Review of Ecology and Systematics, 23 (1): 237–261; doi:10.1146/annurev.es.23.110192.001321
Avise, J. C. (2000) Phylogeography: The History and Formation of Species. Harvard University Press, Cambridge, MA, 464 pp.
Avise, J. C. (2004) Molecular Markers, Natural History, and Evolution. Second edition. Sinauer Associates, Inc. Sunderland, MA, 684pp.
Balshine, S., Leach, B., Neat, F., Reid, H., Taborsky, M. and Werner, N. (2001) Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher). Behavioral Ecology and Sociobiology, 50 (2): 134–140; doi:10.1007/s002650100343 Available from: http://link.springer.com/article/10.1007/s002650100343 (accessed 21 February 2017)
Balshine-Earn, S., Neat, F. C., Reid, H. and Taborsky, M. (1998) Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behavioral Ecology, 9 (5): 432–438; doi: 10.1093/beheco/9.5.432 Available from: https://academic.oup.com/beheco/article/9/5/432/208674/Paying-to-stay-or-paying-to-breed-Field-evidence PDF: http://beheco.oxfordjournals.org/content/9/5/432.full.pdf+html (accessed 27 February 2017)
Baric, S., Salzburger, W. and Sturmbauer, C. (2003) Phylogeography and evolution of the Tanganyikan cichlid genus Tropheus based upon mitochondrial DNA sequences. Journal of Molecular Evolution, 56 (1): 54–68; doi:10.1007/s00239-002-2380-7 Available from: http://www.academia.edu/2157606/Phylogeography_and_evolution_of_the_Tanganyikan_cichlid_genus_Tropheus_based_upon_mitochondrial_DNA_sequences (accessed 5 March 2017)
Barlow, G. W. (2000) The Cichlid Fishes: Nature’s Grand Experiment in Evolution. First paperback edition, printed in 2002. Basic Books, New York, NY, 335 pp.
Beheregaray, L. B. and Caccone, A. (2007) Cryptic biodiversity in a changing world. Journal of Biology, 6 (4): 9; 5 pp; doi:10.1186/jbiol60 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2373901/ (accessed 8 March 2017)
Brichard, P. (1989) Pierre Brichard’s book of cichlids and all the other fishes of Lake Tanganyika. T. F. H. Publications, Neptune City, NJ, 544 pp.
Brown, D. M., Brenneman, R. A., Koepfli, K.-P., Pollinger, J. P., Milá, B., Georgiadis, N. J., Louis, E. E., Grether, G. F., Jacobs, D. K., Wayne, R. K. (2007) Extensive population genetic structure in the giraffe. BMC Biology, 5 (1): 57; doi: 10.1186/1741-7007-5-57 Available from: http://bmcbiol.biomedcentral.com/articles/10.1186/1741-7007-5-57 (accessed 8 March 2017)
Dierkes, P., Heg, D., Taborsky, M., Skubic, E. and Achmann, R. (2005) Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecology Letters, 8 (9): 968–975; doi: 10.1111/j.1461-0248.2005.00801.x Available from: http://www.researchgate.net/publication/227524578_Genetic_relatedness_in_groups_is_sexspecific_and_declines_with_age_of_helpers_in_a_cooperatively_breeding_cichlid (accessed 1 March 2017)
Dierkes, P., Taborsky, M. and Achmann, R. (2008) Multiple paternity in the cooperatively breeding fish Neolamprologus pulcher. Behavioral Ecology and Sociobiology, 62 (10): 1581–1589; doi: 10.1007/s00265-008-0587-3 Available from (PDF): http://behav.zoology.unibe.ch/sysuif/uploads/files/esh/pdf_online/taborskym/Dierkes_BES2008.pdf (accessed 23 February 2017)
Dierkes, P., Taborsky, M. and Kohler, U. (1999) Reproductive parasitism of broodcare helpers in a cooperatively breeding fish. Behavioral Ecology,10 (5): 510–515; doi: https://doi.org/10.1093/beheco/10.5.510 Available from: https://academic.oup.com/beheco/article/10/5/510/221537/Reproductive-parasitism-of-broodcare-helpers-in-a (accessed 18 February 2017
Doyle, J. J. (1992) Gene trees and species trees: Molecular systematics as one-character taxonomy. Systematic Botany, 17 (1): 144–163; doi: 10.2307/2419070 Available from (PDF): ftp://info-ftp-002.amnh.org/pub/people/jlc/Species%20Concepts/Doyle%20Gene%20vs%20SpeciesTrees.pdf (accessed 23 February 2017)
Duftner, N., Sefc, K. M., Koblmüller, S., Salzburger, W., Taborsky, M. and Sturmbauer, C. (2007) Parallel evolution of facial stripe patterns in the Neolamprologus brichardi/pulcher species complex endemic to Lake Tanganyika. Molecular Phylogenetics and Evolution, 45 (2): 706–715; doi: 10.1016/j.ympev.2007.08.001 Available from (PDF): http://behav.zoology.unibe.ch/sysuif/uploads/files/esh/pdf_online/taborskym/Duftner_MolPhylEvol2007.pdf (accessed 23 February 2017)
Eschmeyer, W. N., Fricke, R. and van der Laan, R. (eds.) (2017) Catalog of Fishes: Genera, Species, References. Online version, updated 1 March 2017. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed 10 March 2017)
Fennessy, J., Bidon, T., Reuss, F., Kumar, V., Elkan, P., Nilsson, M. A., Vamberger, M., Fritz, U. and Axel Janke, A. (2016) Multi-locus analyses reveal four giraffe species instead of one. Current Biology, 26 (18): 2543–2549; 10.1016/j.cub.2016.07.036 Available from: http://www.cell.com/current-biology/fulltext/S0960-9822(16)30787-4 (accessed 8 March 2017)
Gante, H. F. and Salzburger, W. (2012) Evolution: cichlid models on the runaway to speciation. Current Biology, 22 (22): R956–R958; 10.1016/j.cub.2012.09.045 Available from: http://www.cell.com/current-biology/fulltext/S0960-9822(12)01147-5 PDF: http://evolution.unibas.ch/salzburger/pub/N03_Curr_Biol_2012.pdf (accessed 23 February 2017)
GCF [Giraffe Conservation Foundation] (2016a) Genetic analysis uncovers four species of giraffe, not just one. GCF News, 8 September 2016. Available from: https://giraffeconservation.org/2016/09/08/genetic-analysis-uncovers-four-species-giraffe-not-just-one/ (accessed 8 March 2017)
GCF [Giraffe Conservation Foundation] (2016b) IUCN Red List confirms: Giraffe are under threat. GCF News, 8 December 2016. Available from: https://giraffeconservation.org/2016/12/08/iucnredlist-giraffe-vulnerable/ (accessed 8 March 2017)
Herrmann, H.-J. (1985) Lamprologus brichardi und seine nächsten Verwandten. DATZ, 38 (4): 152–155. Alfred Kernen-Verlag, Essen.
Hert, E. (1985) Individual recognition of helpers by the breeders in the cichlid fish Lamprologus brichardi (Poll, 1974). Zeitschrift für Tierpsychologie, 68: 313–325; doi:10.1111/j.1439-0310.1985.tb00132.x Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1439-0310.1985.tb00132.x/abstract (accessed 24 February 2017)
IUCN (2016) New bird species and giraffe under threat – IUCN Red List. IUCN News, Thursday, 8 December 2016. Available from: https://www.iucn.org/news/new-bird-species-and-giraffe-under-threat-%E2%80%93-iucn-red-list (accessed 8 March 2017)
Kalas, K. (1976) Brutpflegehelfer und Polygamie beim afrikanischen Buntbarsch Lamprologus brichardi. Die Naturwissenschaften, 63 (2): 94.
Karlsson, M. (1998) Det förflutnas djup. Ciklidbladet, 31 (5): 26–40. Nordiska Ciklidsällskapet.
Karlsson, M. and Karlsson, M. (2013) Lepidiolamprologus kamambae – A new predatory cichlid from Lake Tanganyika. African Diving Lakesite Articles, 1 (1): 1–24; no. 1. First edition; printed. MMK, Sweden.
Karlsson, M. and Karlsson, M. (2014) A princess draped in gold. African Diving Ltd Facebook, 1 December 2014. Available from: https://www.facebook.com/africandivingltd/ link to post: https://www.facebook.com/africandivingltd/posts/1572687739628920:0 (accessed 1 March 2017)
Karlsson, M. and Karlsson, M. (2015a) Kushangaza at Halembe in Lake Tanganyika, and observations on Brichard’s Tropheus. African Diving Blog, 1 March 2015. Available from: http://blog.africandivingltd.com/2015/03/kushangaza-at-halembe-in-lake.html (accessed 6 March 2017)
Karlsson, M. and Karlsson, M. (2015b) Two new geographical colour variants of Ophthalmotilapia sp. “Whitecap” discovered in Lake Tanganyika. Tanganika Magazyn, 17: 7–15. “Tropheus” Robert Mierzeński, Kraków, Poland.
Karlsson, M. and Karlsson, M. (2016) Tropheus cf. moorii “Murago Tanzania” in focus! Tanganika Magazyn, 18: 73–105. “Tropheus” Robert Mierzeński, Kraków, Poland.
Karlsson, M. and Karlsson, M. (2017a) Scenery and underwater video footage from Lake Tanganyika, accompanied by music composed and performed by Orville Stoeber, with lyrics from 'The Year of the Flood' by Margaret Atwood. African Diving Facebook, 15 March 2017. Available from: https://www.facebook.com/africandivingltd/ link to post [video]: https://www.facebook.com/africandivingltd/videos/1896077477289943/ (accessed 15 March 2017)
Karlsson, M. and Karlsson, M. (2017) Neolamprologus sp. “Cygnus” and the N. savoryispecies group (part 3 of 3). African Diving Ltd Facebook, 7 June 2017. Available from: https://www.facebook.com/africandivingltd/ link to post: https://www.facebook.com/africandivingltd/posts/1935804546650569:0 (accessed 18 June 2017)
Koblmüller, S., Egger, B., Sturmbauer, C. and Sefc, K. (2010) Rapid radiation, ancient incomplete lineage sorting and ancient hybridization in the endemic Lake Tanganyika cichlid tribe Tropheini. Molecular Phylogenetics and Evolution, 55 (1): 318–334; doi: 10.1016/j.ympev.2009.09.032 Available from: https://www.researchgate.net/publication/38032530_Rapid_radiation_ancient_incomplete_lineage_sorting_and_ancient_hybridization_in_the_endemic_Lake_Tanganyika_cichlid_tribe_Tropheini (accessed 3 November 2016)
Kohda, M., Jordan, L. A., Hotta, T., Kosaka, N., Karino, K., Tanaka, H., Taniyama, M. and Takeyama, T. (2015) Facial recognition in a group-living cichlid. PLoS ONE, 10 (11): e0142552; 12 pp; doi:10.1371/journal.pone.0142552 Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0142552 (accessed 23 February 2017)
Konings, A. (1998) Tanganyika cichlids in their natural habitat. Cichlid Press, El Paso, TX, 272 pp.
Konings, A. (2015) Tanganyika cichlids in their natural habitat. 3rd edition. Cichlid Press, El Paso, TX, 408 pp.
Kullander, S. O. (2004) Species, subspecies, and such. Pp. 4–21 in: Gessner, J. and Ritterhoff, J. (eds.) Species differentiation and population identification in the sturgeons Acipenser sturio L. and Acipenser oxyrhinchus. BfN-Skripten, 101. Federal Agency for nature Conservation.
Kullander, S. O., Karlsson, M., Karlsson, M. and Norén, M. (2014b) Chalinochromis cyanophleps, a new species of cichlid fish (Teleostei: Cichlidae) from Lake Tanganyika. Zootaxa, 3790 (3): 425–438. Available from (PDF): http://mapress.com/zootaxa/2014/f/zt03790p438.pdf (accessed 23 February 2017)
Kullander, S. O., Norén, M., Karlsson, M. and Karlsson, M. (2014a) Description of Neolamprologus timidus, new species, and review of N. furcifer from Lake Tanganyika (Teleostei: Cichlidae). Ichthyological Explorations of Freshwaters, 24 (4): 301–328. Available from (PDF): http://pfeil-verlag.de/wp-content/uploads/2017/04/ief24_4_03.pdf (accessed 23 February 2017)
Kullander, S. O., Norén, M., Karlsson, M. and Karlsson, M. (2014a) Description of Neolamprologus timidus, new species, and review of N. furcifer from Lake Tanganyika (Teleostei: Cichlidae). Ichthyological Explorations of Freshwaters, 24 (4): 301–328. Available from (PDF): http://pfeil-verlag.de/wp-content/uploads/2017/04/ief24_4_03.pdf (accessed 23 February 2017)
Maddison, W. P. (1997) Gene trees in species trees. Systematic Biology, 46 (3): 523–536; doi: 10.1093/sysbio/46.3.523 Available from: http://sysbio.oxfordjournals.org/content/46/3/523.abstract (accessed 23 February 2017)
Maréchal, C. and Poll, M. (1991) In: Daget, J., Gosse, J.-P., Teugels, G. G. and Thys van den Audenaerde, D. F. E. (eds.) Check-list of freshwater fishes of Africa (CLOFFA), vol. 4. ISNB, Brussels, MRAC, Tervuren, ORSTOM, Paris, 740 pp.
Muller, Z., Bercovitch, F., Brand, R., Brown, D., Brown, M., Bolger, D., Carter, K., Deacon, F., Doherty, J. B., Fennessy, J., Fennessy, S., Hussein, A. A., Lee, D., Marais, A., Strauss, M., Tutchings, A. and Wube, T. (2016) Giraffa camelopardalis. The IUCN Red List of Threatened Species 2016: e.T9194A51140239; doi: 10.2305/IUCN.UK.2016-3.RLTS.T9194A51140239.en Available from: http://www.iucnredlist.org/details/9194/0 (accessed 8 March 2017)
Nevado, B., Fazzalova, V., Backeljau, T., Hanssens, M. and Verheyen, E. (2011) Repeated unidirectional introgression of nuclear and mitochondrial DNA between four congeneric Tanganyikan cichlids. Molecular Biology and Evolution, 28 (8): 2253–2267; 10.1093/molbev/msr043 Available from: http://mbe.oxfordjournals.org/content/28/8/2253.full (accessed 21 February 2017)
Pamilo, P. and Nei, M. (1988) Relationships between gene trees and species trees. Molecular Biology and Evolution, 5 (5): 568–583; doi: 10.1093/oxfordjournals.molbev.a040517 Available from: http://www.ncbi.nlm.nih.gov/pubmed/3193878 (accessed 23 February 2017)
Poll, M. (1974) Contribution à la faune ichthyologique du lac Tanganika, d'après les récoltes de P. Brichard. Revue de Zoologie africaine, 88 (1): 99–110.
Salzburger, W., Baric, S. and Sturmbauer, C. (2002) Speciation via introgressive hybridization in East African cichlids? Molecular Ecology, 11 (3): 619–625; doi: 10.1046/j.0962-1083.2001.01438.x Available from: http://www.ncbi.nlm.nih.gov/pubmed/11918795 (accessed 23 December 2016)
Seehausen, O. (2006) African cichlid fish: a model system in adaptive radiation research. Proceedings of the Royal Society B: Biological Sciences, 273 (1597): 1987–1998; doi: 10.1098/rspb.2006.3539 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1635482/ (accessed 21 February 2017)
Smith, M. (1995) Current status of Lake Tanganyika’s “Lamprologus”. Aquarium Frontiers, 2 (4): 4–5, 16–19.
Stiassny, M. L. J. (1997) A phylogenetic overview of the lamprologine cichlids of Africa (Teleostei, Cichlidae): A morphological perspective. South African Journal of Science, 93 (11–12): 513–523.
Stiver, K. A., Dierkes, P., Taborsky, M. and Balshine, S. (2004) Dispersal patterns and status change in a co-operatively breeding cichlid Neolamprologus pulcher: evidence from microsatellite analyses and behavioural observations. Journal of Fish Biology, 65 (1): 91–105; doi: 10.1111/j.0022-1112.2004.00427.x Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.0022-1112.2004.00427.x/abstract (accessed 4 March 2017)
Streelman, J. T., Zardoya, R., Meyer, A. and Karl, S. A. (1998) Multilocus phylogeny of cichlid fishes (Pisces: Perciformes): evolutionary comparison of microsatellite and single-copy nuclear loci. Molecular Biology and Evolution, 15 (7): 798–808. Available from (PDF): http://mbe.oxfordjournals.org/content/15/7/798.full.pdf (accessed 1 March 2017)
Sturmbauer, C. and Meyer, A. (1992) Genetic divergence, speciation and morphological stasis in a lineage of African cichlid fishes. Nature, 358 (6387): 578–581; doi:10.1038/358578a0 Available from: https://www.researchgate.net/publication/21653433_Genetic_divergence_speciation_and_morphological_stasis_in_a_lineage_of_African_cichlid_fishesn (accessed 5 March 2017)
Sturmbauer, C., Koblmüller, S., Sefc, K. M. and Duftner, N. (2005) Phylogeographic history of the genus Tropheus, a lineage of rock-dwelling cichlid fishes endemic to Lake Tanganyika. Hydrobiologia, 542 (1–3): 335–366; doi:10.1007/s10750-004-4664-y Available from: https://www.researchgate.net/publication/225037160_Phylogeographic_history_of_the_genus_Tropheus_a_lineage_of_rock-dwelling_cichlid_fishes_endemic_to_Lake_Tanganyika (accessed 5 March 2017)
Sturmbauer, C., Salzburger, W., Duftner, N., Schelly, R. and Koblmüller, S. (2010) Evolutionary history of the Lake Tanganyika cichlid tribe Lamprologini (Teleostei: Perciformes) derived from mitochondrial and nuclear DNA data. Molecular Phylogenetics and Evolution, 57 (1): 266–284; doi: 10.1016/j.ympev.2010.06.018 Available from: http://www.sciencedirect.com/science/article/pii/S1055790310002897 (accessed 1 March 2017)
Tibbetts, E. A. and Dale, J. (2007) Individual recognition: it is good to be different. Trends in Ecology and Evolution, 22 (10): 529–537; doi: 10.1016/j.tree.2007.09.001 Available from: http://www.cell.com/trends/ecology-evolution/abstract/S0169-5347(07)00237-6 (accessed 25 February 2016)
Trewavas, E. and Poll, M. (1952) Three new species and two new subspecies of the genus Lamprologus, Cichlid fishes of Lake Tanganyika. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, 28 (50): 1–16.
Verburg, P. and Bills, R. (2007) Two new cichlid species Neolamprologus (Teleostei: Cichlidae) from Lake Tanganyika, East Africa. Zootaxa, 1612: 25–44.



























